Hot off the *New England Journal of Medicine (NEJM)*press is a new article reporting on the results of a randomized trial of the drug remdesivir in COVID-19 patients. Bottom line: the trial shows the drug is effective both in shortening duration and lowering mortality in patients who are started on treatment before they need a ventilator.
Let’s put this in context. If you are looking for some background on the science and more about treatment in general, this is a great time to head over to our Treatment Explainer.
What is Remdesivir?
Remdesivir is an antiviral drug which works by blocking viral RNA so it cannot replicate. This drug existed before COVID-19. Because it relies on a mechanism common to a lot of RNA viruses, researchers had been studying its effectiveness in a number of viruses before SARS-CoV2 came along. Originally designed as a treatment for Hepatitis C and then repurposed for Ebola trials, it has since been shown to be effective against the original SARS and MERS (Middle East Respiratory Syndrome). Because SARS-CoV2 (the virus which causes COVID-19) is related to SARS and MERS, this was identified early on as a possible candidate drug to fight COVID-19.
Why is a randomized trial valuable?
This new NEJM study reports results from a *randomized trial.*This means that this study was conducted by enrolling patients with COVID-19 and randomly choosing some of them to receive doses of Remdesivir while others received the placebo (basically, a sugar pill). The trial enrolled slightly over 1000 people: 541 received remdesivir and 522 received the placebo.
The randomization is key. Because those who received remdesivir were chosen randomly, we can be confident they are not systematically different in other ways than those who got the placebo (i.e. we can be sure that both groups had roughly the same composition of people in terms of gender, age, and medical risk factors.). This type of randomized trial is considered to be the “gold standard” in evidence.
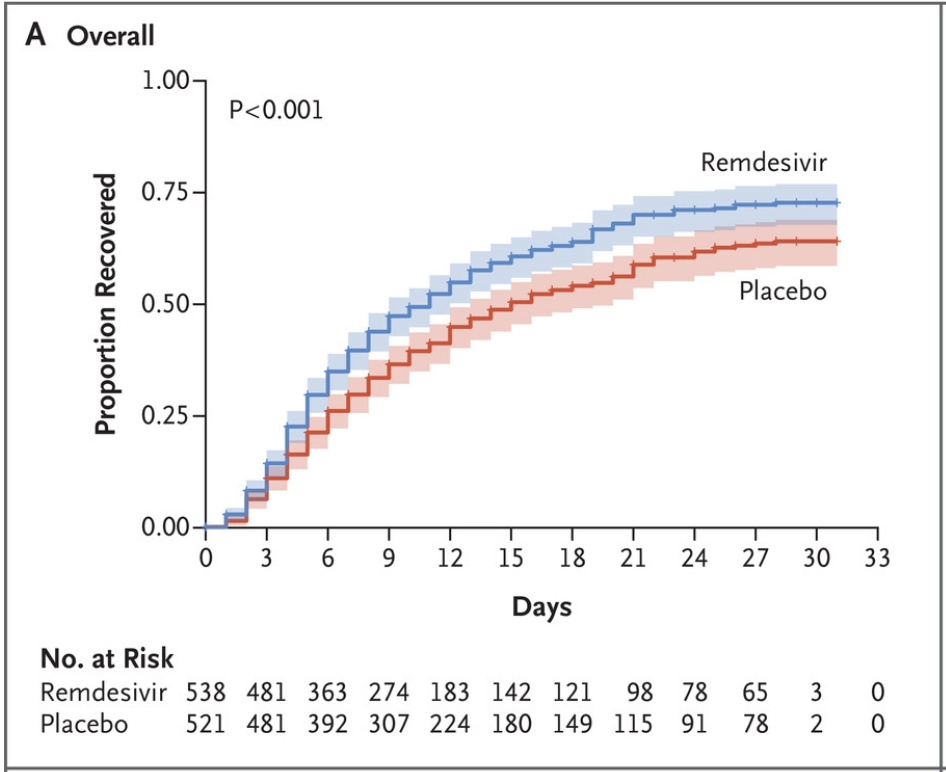
What did the trial find?
The trial found evidence for the efficacy of remdesivir. The two key findings are a shorter recovery time (11 days versus 15 days) and lower mortality at 14 days (7% versus 12%). We’ve included the key figure showing recovery rates below. The main thing to see is that the proportion recovered is higher at most time scales for the remdesivir group than the placebo group.
The effects were so big the study was stopped early. The data safety and monitoring board (commonly used in these sorts of trials) is a group of experts not involved in the trial tasked with peeking in on the data at certain points to see if anything really good or really bad is happening. They felt these data were so strong that they advised stopping the trial so everyone could benefit.
This trial also allowed doctors to start to see who had the most benefit from the drug. Patients who were hospitalized, or on oxygen, showed a large improvement. This effect begins to decline, however, in patients that were on more heavy duty forms of oxygen therapy, including being intubated (a breathing tube put into their lungs).
We should be clear: this isn’t a “cure.” The mortality rates in this (high risk) population are still high even in the remdesivir group. But the effects are very large. Scaled up to the infected population, this is a potentially large gain in lives saved.
What do we still need to know?
Lots. There are other candidate treatments, including other antivirals, so it will be useful to see if those drugs work better or worse. Even with remdesivir, we would still like to know whether it works better earlier in the course of the disease or later, whether some patients benefit more, what the optimal dose is, and how big the decrease in mortality is.
